Catalytic Generator: Difference between revisions
Adds general info and cathode efficacy matrix |
Niftyspigots (talk | contribs) added department guide template |
||
| (5 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
''See Also: [[Power_Grid|Power Grid]]'' | |||
[[File:NadirCatalyticEngineSouth.png|512px]] | [[File:NadirCatalyticEngineSouth.png|512px]] | ||
| Line 6: | Line 7: | ||
===Making Rods=== | ===Making Rods=== | ||
Rods can be manufactured at a [[Ore_Processing#The_Nano-Fabricator_.28Refining.29|refining nano-fabricator]], which can be found in the refinery or in engineering. They require one unit of a material, and the material must be metallic. The material properties of the rod determine its effectiveness as a cathode or an anode. Furthermore, the material's chemical resistance determines how rapidly it degrades over time. | Rods can be manufactured at a [[Ore_Processing#The_Nano-Fabricator_.28Refining.29|refining nano-fabricator]], which can be found in the refinery or in engineering. They require one unit of a material, and the material must be metallic. The material properties of the rod determine its effectiveness as a cathode or an anode. Furthermore, the material's chemical resistance determines how rapidly it degrades over time. In order to obtain materials, one could use the [[Extraction_Nexus|Harmonic Siphon]], which both [[engineer]]s and [[scientist]]s can access. Alternatively, [[engineer]]s have access to mining tools and may to descend into the trench and mine ores the old-fashioned way. | ||
===Simple Output | ===Simple Output Formula=== | ||
The output of a catalytic engine is the lesser of the output power of the cathode and anode rods. The output power of a rod is determined by its condition (a number | The output of a catalytic engine is the lesser of the output power of the cathode and anode rods. The output power of a rod is determined by its condition (a number that starts at 100 and progresses towards 0 as the rod corrodes) multiplied by its efficacy (a positive number that represents how effective the materials are) multiplied by ten. Using this formula with the best possible rod formulations and perfect rod condition, we find that the maximum possible engine output is 977 kilowatts. | ||
===Cathode Efficacy=== | ===Cathode Efficacy=== | ||
The efficacy of the cathode rod is determined by its hardness and density. Optimally, the cathode has 5 density and 5 hardness. The efficacy formula places more importance on density than hardness, as detailed in the | The efficacy of the cathode rod is determined by its hardness and density. Optimally, the cathode has 5 density and 5 hardness. The efficacy formula places more importance on density than hardness, as detailed in the tables below: | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 40: | Line 41: | ||
|} | |} | ||
===Anode=== | {| class="wikitable" | ||
|+ Material Cathode Effectiveness | |||
|- | |||
! Material !! Density !! Hardness !! Cathode Efficacy !! Chemical Resistance | |||
|- | |||
| '''Plasmasteel + Plasmaglass''' || '''5''' || '''5''' || '''1055''' || '''5''' | |||
|- | |||
| Bohrum || 6 || 5 || 332 || 7 | |||
|- | |||
| Mauxite || 4 || 3 || 111 || 5 | |||
|- | |||
| Steel || 4 || 3 || 111 || 5 | |||
|} | |||
===Anode Efficacy=== | |||
The efficacy of the anode rod is determined by its electrical conductivity and whether it is an energy source or not. Optimally, the anode has 9 electrical and is an energy source. The details are listed in the tables below: | |||
{| class="wikitable" | |||
|+ Anode Efficacy | |||
|- | |||
! Electrical !! Normal !! Energy Source | |||
|- | |||
| '''1''' || 17 || 22 | |||
|- | |||
| '''2''' || 34 || 44 | |||
|- | |||
| '''3''' || 51 || 66 | |||
|- | |||
| '''4''' || 68 || 88 | |||
|- | |||
| '''5''' || 85 || 149 | |||
|- | |||
| '''6''' || 108 || 309 | |||
|- | |||
| '''7''' || 201 || 573 | |||
|- | |||
| '''8''' || 342 || 977 | |||
|- | |||
| '''9''' || 548 || '''1565''' | |||
|} | |||
{| class="wikitable" | |||
|+ Material Anode Effectiveness | |||
|- | |||
! Material !! Electrical !! Energy Source? !! Anode Efficacy !! Chemical Resistance | |||
|- | |||
| '''Electrum +<br> Cerenkite +<br> Electrum''' || '''8''' || '''Yes''' || '''977''' || '''6''' | |||
|- | |||
| Electrum +<br> Cerenkite || 7 || Yes || 573 || 6 | |||
|- | |||
| Claretine +<br> Cerenkite || 7 || Yes || 573 || 5 | |||
|- | |||
| Electrum || 9 || No || 548 || 6 | |||
|- | |||
| Cerenkite || 6 || Yes || 309 || 6 | |||
|- | |||
| Pharosium +<br> Cerenkite || 6 || Yes || 309 || 6 | |||
|- | |||
| Pharosium || 7 || No || 201 || 6 | |||
|- | |||
| Claretine +<br> Pharosium || 7 || No || 201 || 5 | |||
|- | |||
| Copper || 6 || No || 108 || 6 | |||
|} | |||
Due to not being a metal, pure claretine is not useable as an anode rod. Fractional stats of alloys are rounded down, meaning that some combinations that might intuitively improve the rod efficacy actually do not. It is currently impossible to make a material that has 9 electrical and is an energy source. | |||
===Corrosion Resistance=== | |||
Anode and cathode rods are corroded as they generate power. This occurs by multiplying their condition by a decay ratio every time the power grid is calculated. The decay ratio is determined by chemical resistance, as shown in the following table: | |||
{| class="wikitable" | |||
|+ Decay Ratios | |||
|- | |||
! Chemical Resistance !! Decay Ratio | |||
|- | |||
| 1 || 0.99823 | |||
|- | |||
| 2 || 0.99846 | |||
|- | |||
| 3 || 0.99869 | |||
|- | |||
| 4 || 0.99892 | |||
|- | |||
| 5 || 0.99915 | |||
|- | |||
| 6 || 0.99938 | |||
|- | |||
| 7 || 0.99961 | |||
|- | |||
| 8 || 0.99984 | |||
|- | |||
| '''9''' || '''1''' | |||
|} | |||
Note that a chemical resistance of 9 means that the rod does not decay over time at all. There are currently 3 materials that have a chemical resistance of 9, being uqill, dyneema, and iridium. Of the 3, only iridium is a metal, and its stats as a cathode or anode are quite poor (29 cathode efficacy, 85 anode efficacy). | |||
---- | |||
{{Department Guides}} | |||
Latest revision as of 09:22, 12 January 2023
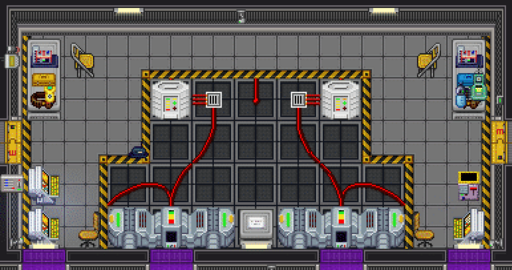
See Also: Power Grid
The catalytic generators are the main source of power for Nadir. They generate power by exposing cathode and anode rods to the acid sea, forming ions which can be used to generate electrical power. However, the rods degrade over time and will need to be periodically replaced to maintain power output. The anode rod goes in the left side of the engine, and the cathode rod goes in the right side. There are four engines, two in the northernmost and southernmost areas of the station.
Making Rods
Rods can be manufactured at a refining nano-fabricator, which can be found in the refinery or in engineering. They require one unit of a material, and the material must be metallic. The material properties of the rod determine its effectiveness as a cathode or an anode. Furthermore, the material's chemical resistance determines how rapidly it degrades over time. In order to obtain materials, one could use the Harmonic Siphon, which both engineers and scientists can access. Alternatively, engineers have access to mining tools and may to descend into the trench and mine ores the old-fashioned way.
Simple Output Formula
The output of a catalytic engine is the lesser of the output power of the cathode and anode rods. The output power of a rod is determined by its condition (a number that starts at 100 and progresses towards 0 as the rod corrodes) multiplied by its efficacy (a positive number that represents how effective the materials are) multiplied by ten. Using this formula with the best possible rod formulations and perfect rod condition, we find that the maximum possible engine output is 977 kilowatts.
Cathode Efficacy
The efficacy of the cathode rod is determined by its hardness and density. Optimally, the cathode has 5 density and 5 hardness. The efficacy formula places more importance on density than hardness, as detailed in the tables below:
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 23 | 29 | 34 | 40 | 45 | 40 | 34 | 29 | 23 |
| 3 | 47 | 58 | 68 | 79 | 90 | 79 | 68 | 58 | 47 |
| 4 | 70 | 86 | 111 | 199 | 332 | 199 | 111 | 86 | 70 |
| 5 | 94 | 176 | 350 | 630 | 1050 | 630 | 350 | 176 | 94 |
| 6 | 70 | 86 | 111 | 199 | 332 | 199 | 111 | 86 | 70 |
| 7 | 47 | 58 | 68 | 79 | 90 | 79 | 68 | 58 | 47 |
| 8 | 23 | 29 | 34 | 40 | 45 | 40 | 34 | 29 | 23 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Material | Density | Hardness | Cathode Efficacy | Chemical Resistance |
|---|---|---|---|---|
| Plasmasteel + Plasmaglass | 5 | 5 | 1055 | 5 |
| Bohrum | 6 | 5 | 332 | 7 |
| Mauxite | 4 | 3 | 111 | 5 |
| Steel | 4 | 3 | 111 | 5 |
Anode Efficacy
The efficacy of the anode rod is determined by its electrical conductivity and whether it is an energy source or not. Optimally, the anode has 9 electrical and is an energy source. The details are listed in the tables below:
| Electrical | Normal | Energy Source |
|---|---|---|
| 1 | 17 | 22 |
| 2 | 34 | 44 |
| 3 | 51 | 66 |
| 4 | 68 | 88 |
| 5 | 85 | 149 |
| 6 | 108 | 309 |
| 7 | 201 | 573 |
| 8 | 342 | 977 |
| 9 | 548 | 1565 |
| Material | Electrical | Energy Source? | Anode Efficacy | Chemical Resistance |
|---|---|---|---|---|
| Electrum + Cerenkite + Electrum |
8 | Yes | 977 | 6 |
| Electrum + Cerenkite |
7 | Yes | 573 | 6 |
| Claretine + Cerenkite |
7 | Yes | 573 | 5 |
| Electrum | 9 | No | 548 | 6 |
| Cerenkite | 6 | Yes | 309 | 6 |
| Pharosium + Cerenkite |
6 | Yes | 309 | 6 |
| Pharosium | 7 | No | 201 | 6 |
| Claretine + Pharosium |
7 | No | 201 | 5 |
| Copper | 6 | No | 108 | 6 |
Due to not being a metal, pure claretine is not useable as an anode rod. Fractional stats of alloys are rounded down, meaning that some combinations that might intuitively improve the rod efficacy actually do not. It is currently impossible to make a material that has 9 electrical and is an energy source.
Corrosion Resistance
Anode and cathode rods are corroded as they generate power. This occurs by multiplying their condition by a decay ratio every time the power grid is calculated. The decay ratio is determined by chemical resistance, as shown in the following table:
| Chemical Resistance | Decay Ratio |
|---|---|
| 1 | 0.99823 |
| 2 | 0.99846 |
| 3 | 0.99869 |
| 4 | 0.99892 |
| 5 | 0.99915 |
| 6 | 0.99938 |
| 7 | 0.99961 |
| 8 | 0.99984 |
| 9 | 1 |
Note that a chemical resistance of 9 means that the rod does not decay over time at all. There are currently 3 materials that have a chemical resistance of 9, being uqill, dyneema, and iridium. Of the 3, only iridium is a metal, and its stats as a cathode or anode are quite poor (29 cathode efficacy, 85 anode efficacy).
| Department Guides | |
|---|---|
| Engineering | Making and Breaking · Construction · Gas · Power Grid · Thermoelectric Generator · Singularity Generator · Geothermal Generator · Catalytic Generator · Nuclear Generator · Mining · Materials and Crafting · Wiring · Hacking · MechComp · Mechanic components and you · Control Unit · Ruckingenur Kit · Reactor Statistics Computer · Cargo Crates |
| Medsci | Doctoring · Genetics · Robotics · Telescience · Plasma Research · Artifact Research · Chemistry · Chemicals · ChemiCompiler · Decomposition |
| Security | Security Officer · Contraband · Forensics · Space Law |
| Service | Foods and Drinks · Botany · Writing · Piano Song Dump · Instruments |
| The AI | Artificial Intelligence · AI Laws · Chain of Command · Guide to AI · Humans and Nonhumans · Killing the AI |
| Computers | Computers · TermOS · ThinkDOS · Packets |